
(简答题)
已知: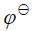 (Ag+/Ag) = 0.7991V,
(Ag+/Ag) = 0.7991V,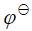 (AgBr/Ag) = 0.071V,
(AgBr/Ag) = 0.071V, (Zn2+/Zn) = -0.763V,
(Zn2+/Zn) = -0.763V, 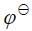 (Ag(S2O3)3-2/Ag) = 0.010V 确定0.0010mol AgBr能否溶于100cm3 0.025 mol·dm-3的Na2S2O3溶液中(生成Br-和Ag(S2O3)3-2);
(Ag(S2O3)3-2/Ag) = 0.010V 确定0.0010mol AgBr能否溶于100cm3 0.025 mol·dm-3的Na2S2O3溶液中(生成Br-和Ag(S2O3)3-2);
正确答案

答案解析
略
相似试题
(简答题)
已知:(Ag+/Ag) = 0.7991V,(AgBr/Ag) = 0.071V,(Zn2+/Zn) = -0.763V, (Ag(S2O3)3-2/Ag) = 0.010V
(简答题)
已知:(Ag+/Ag) = 0.7991V,(AgBr/Ag) = 0.071V,(Zn2+/Zn) = -0.763V, (Ag(S2O3)3-2/Ag) = 0.010V
(简答题)
已知:(Ag+/Ag) = 0.7991V,(AgBr/Ag) = 0.071V,(Zn2+/Zn) = -0.763V, (Ag(S2O3)3-2/Ag) = 0.010V
(简答题)
已知:(Ag+/Ag) = 0.7991V,(AgBr/Ag) = 0.071V,(Zn2+/Zn) = -0.763V, (Ag(S2O3)3-2/Ag) = 0.010V
(简答题)
设计电池,计算298 K时AgCl的溶度积。已知υөAgCl,Ag=0.2223V,υөAg+,Ag=0.7994V。
(简答题)
已知下述电池的电动势为0.18V.已知:φ°(Ag+/Ag)=0.7996V φ°(Cu2+/Cu)=0.34V. 试计算AgBr的Ksp。(-)Ag│AgBr([Br]=0.1mol/L)‖Cu2+(0.1mol/L)│Cu (+)
(简答题)
已知Eθ(Ag+/Ag)和Eθ(Fe3+/Fe2+)分别为0.799V和0.771V。下列原电池:
(单选题)
已知φ0(Ag+,Ag)=0.799V,φ0(Pb2+,Pb)=-0.126V,在298K、p0下,电解含Ag+、Pb2+活度各为1的溶液,当Pb2+离子开始析出时,Ag+的浓度是:()
(单选题)
已知φθAg+/Ag=0.80V,φθZn2+/Zn=-0.76V。将两电对组成原电池,该原电池的标准电动势为()


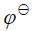 (Ag+/Ag) = 0.7991V,
(Ag+/Ag) = 0.7991V,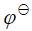 (AgBr/Ag) = 0.071V,
(AgBr/Ag) = 0.071V, (Zn2+/Zn) = -0.763V,
(Zn2+/Zn) = -0.763V, 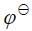 (Ag(S2O3)3-2/Ag) = 0.010V 确定0.0010mol AgBr能否溶于100cm3 0.025 mol·dm-3的Na2S2O3溶液中(生成Br-和Ag(S2O3)3-2);
(Ag(S2O3)3-2/Ag) = 0.010V 确定0.0010mol AgBr能否溶于100cm3 0.025 mol·dm-3的Na2S2O3溶液中(生成Br-和Ag(S2O3)3-2); 